VEMLIDY trial participants were studied through 8 years (Week 384)
Trial 108 (425 patients) and Trial 110 (873 patients) studied the efficacy and safety of VEMLIDY (25 mg daily) for patients with chronic hepatitis B with stable liver disease compared to another medicine called tenofovir disoproxil fumarate (TDF, 300 mg daily).
Between these 2 trials, patients were either on VEMLIDY (866 patients) or TDF (432 patients) until Week 96. At the end of 2 years, most adults who had been taking VEMLIDY continued, and many who were taking TDF switched to VEMLIDY. Patients in this clinical trial were followed until Week 384 (1298 patients).
Hepatitis B can be more active or less active in different people:
- HBeAg- (negative) means the virus is less active, so it doesn’t spread as much
- HBeAg+ (positive) means there was a recent infection, making the virus easier to spread to others
Trial 108 included patients with less active hepatitis B (HBeAg-). Trial 110 included patients with more active hepatitis B (HBeAg+).
As of April 25, 2024, Trial 110 was one of the largest, well-controlled studies of patients with chronic hepatitis B.

HBeAg=hepatitis B envelope antigen.
VEMLIDY was shown to reduce the amount of hepatitis B virus in patients
The studies looked at hepatitis B virus levels in the body, which is called HBV DNA viral load. With hepatitis B, the amount of viral load can go up or down. A higher viral load can lead to more serious effects of chronic hepatitis B.
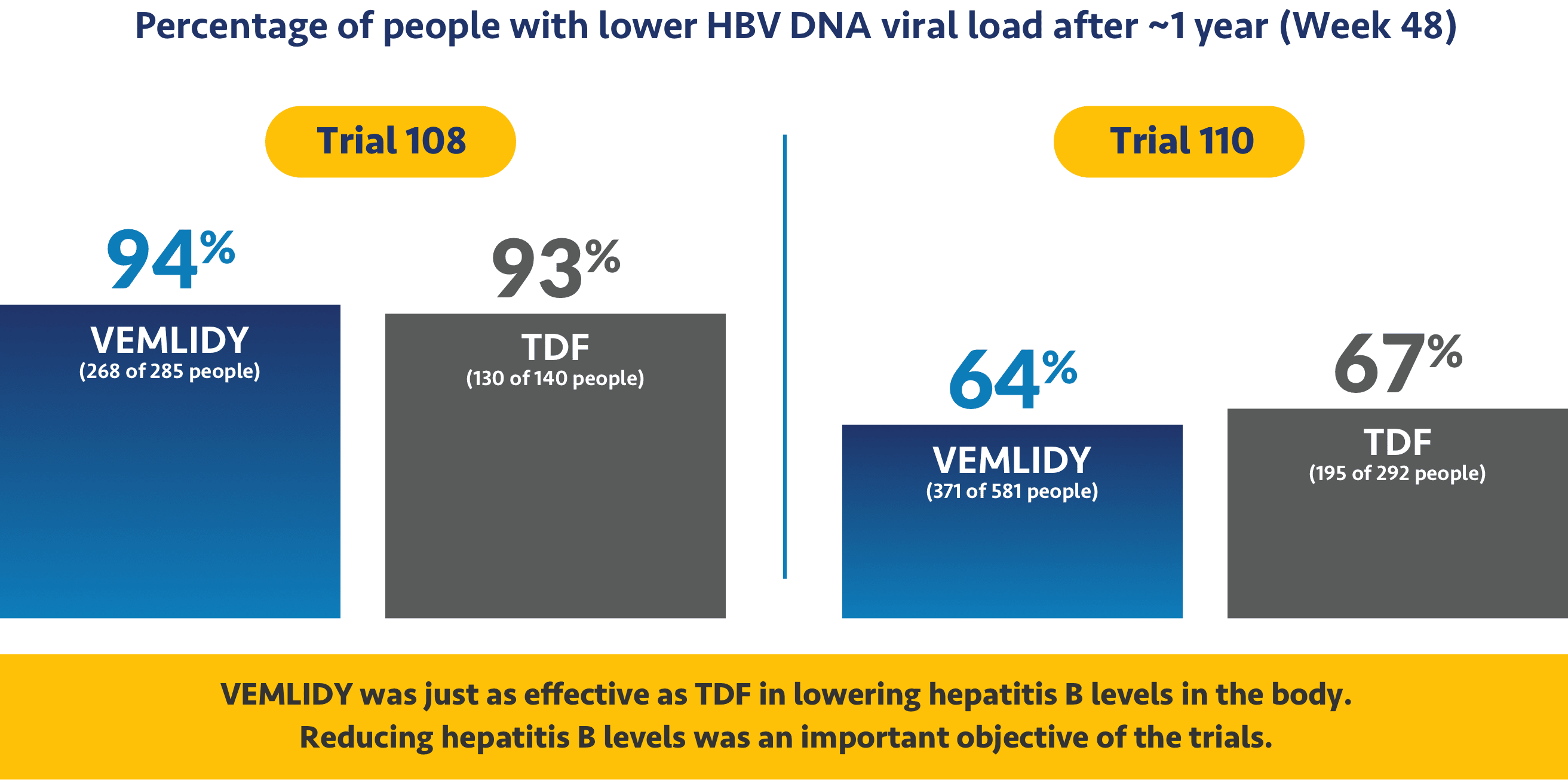

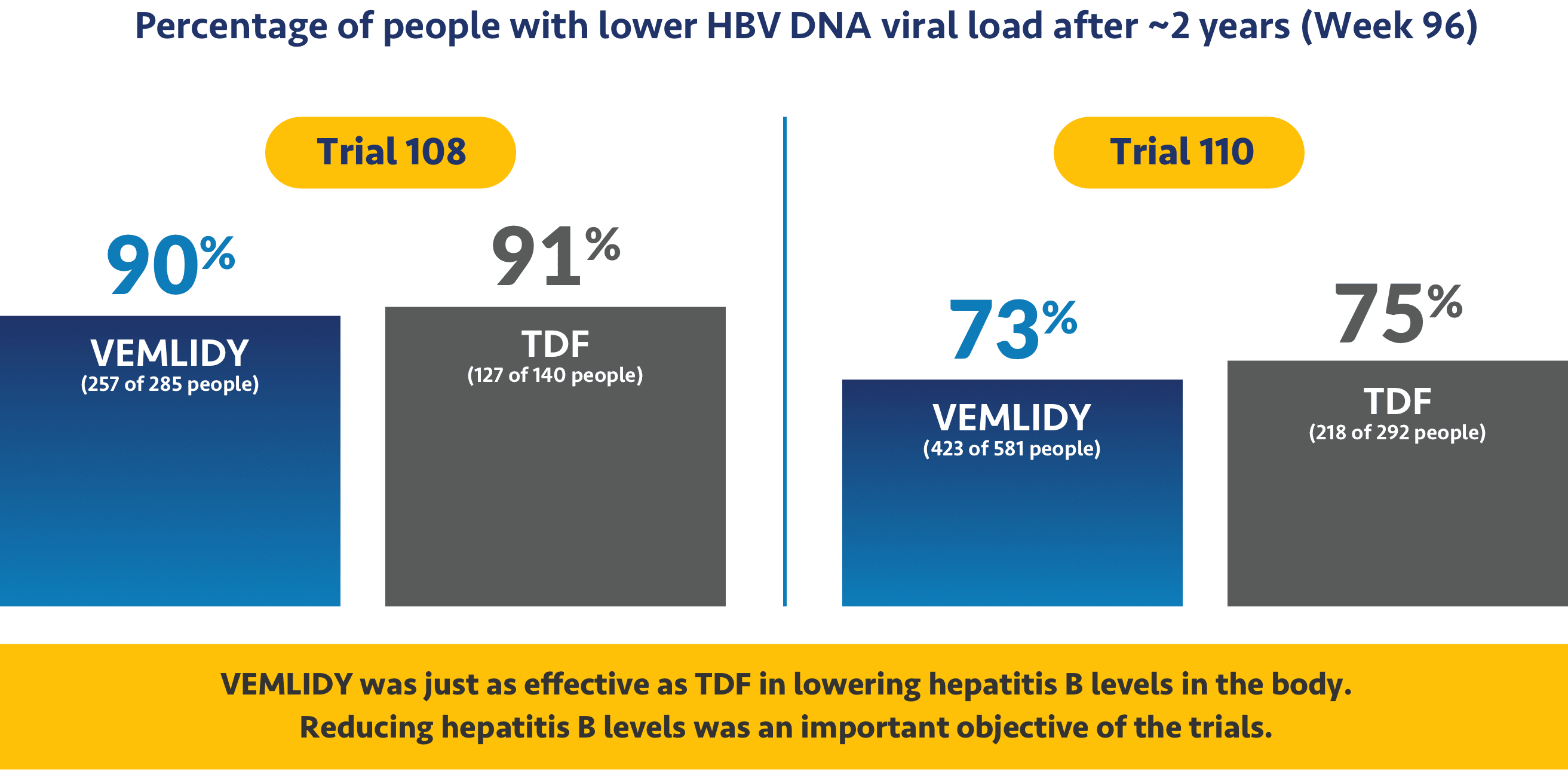
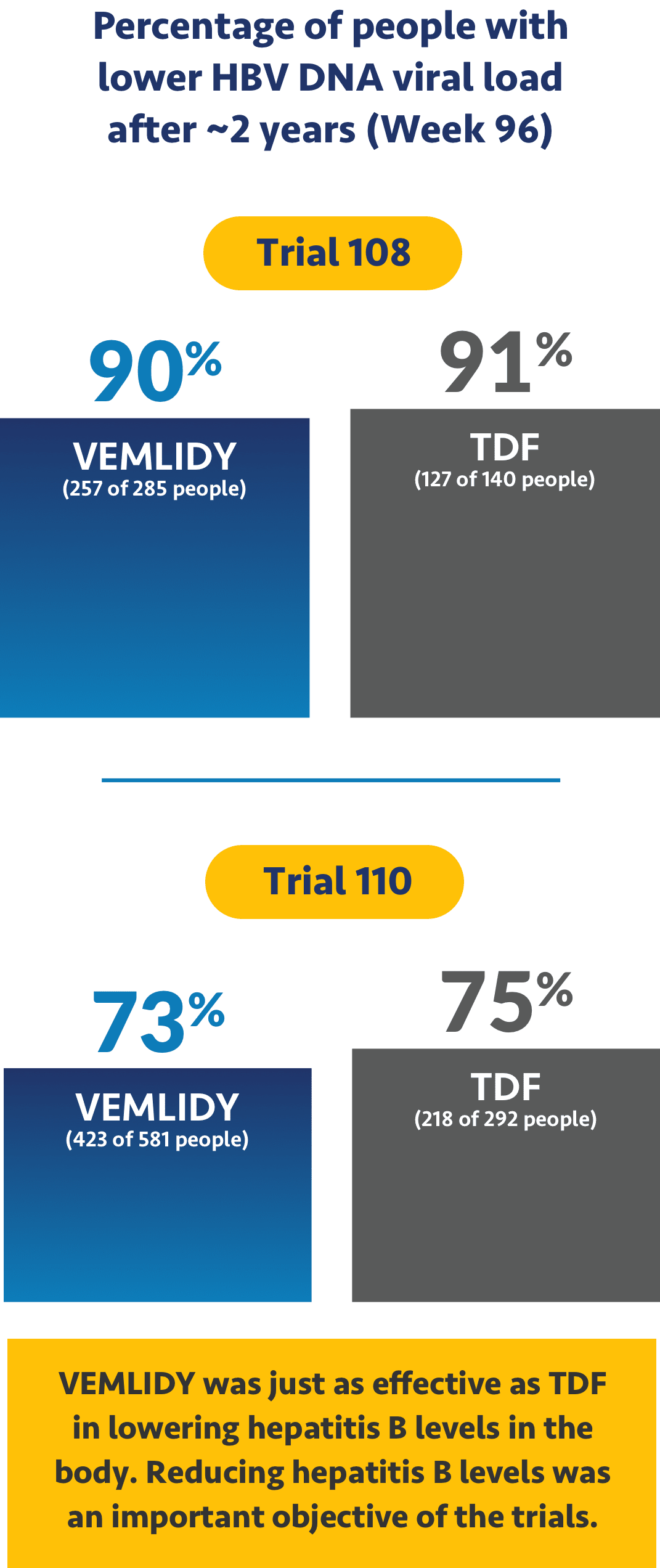
Ask your doctor about additional data points up to 8 years (Week 384).
The most common side effects (in 5% or more patients) in clinical trials were headache, stomach discomfort, cough, back pain, fatigue, nausea, arthralgia (joint pain), diarrhea, and dyspepsia (indigestion).
Impact on ALT levels
The studies also looked to see if treatment helped levels of alanine aminotransferase, or ALT, return to normal. ALT is a measure of liver damage, so achieving normalized ALT levels was an objective of the trials.
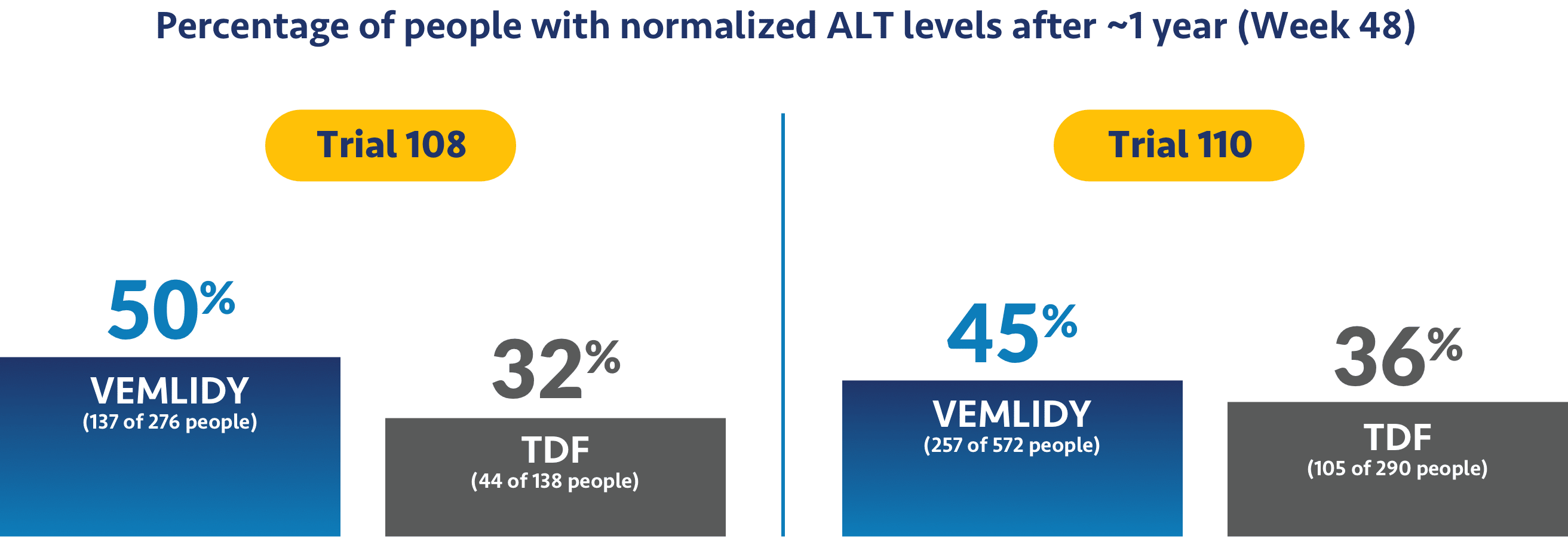
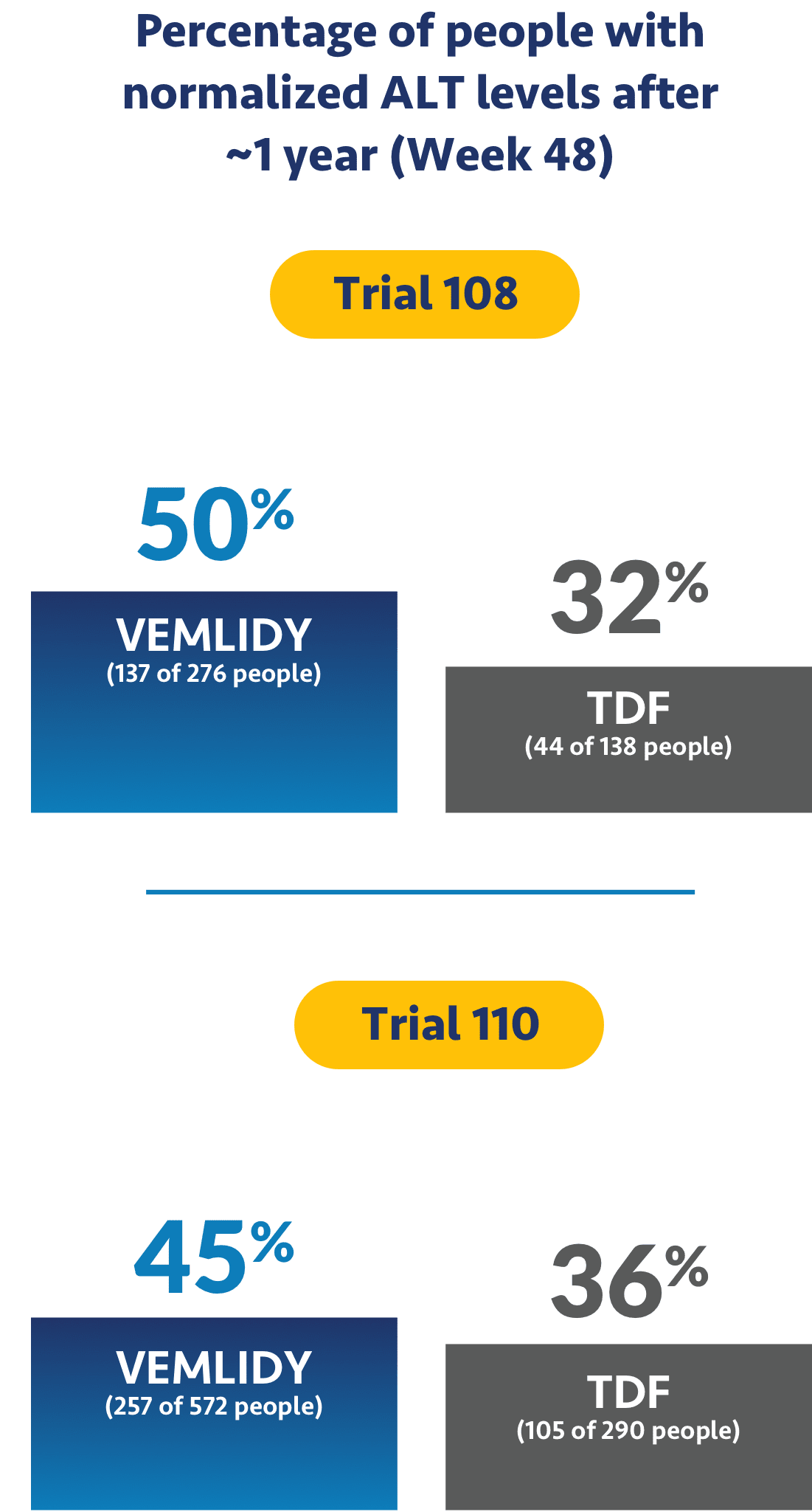
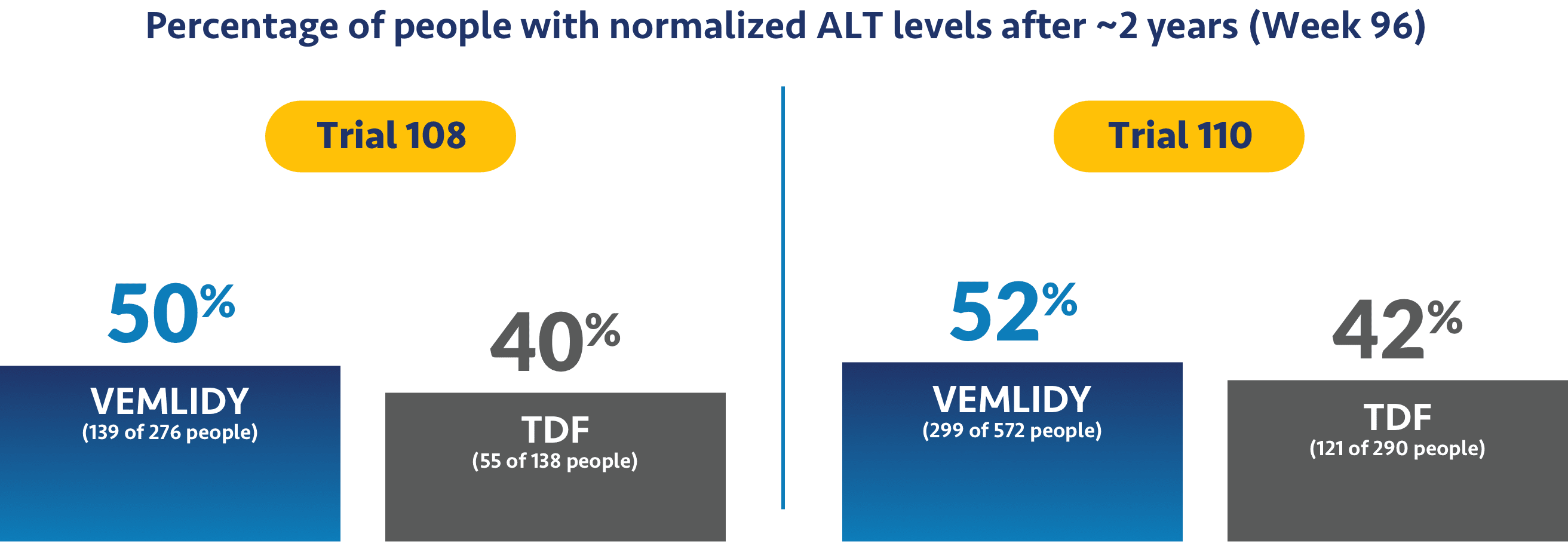
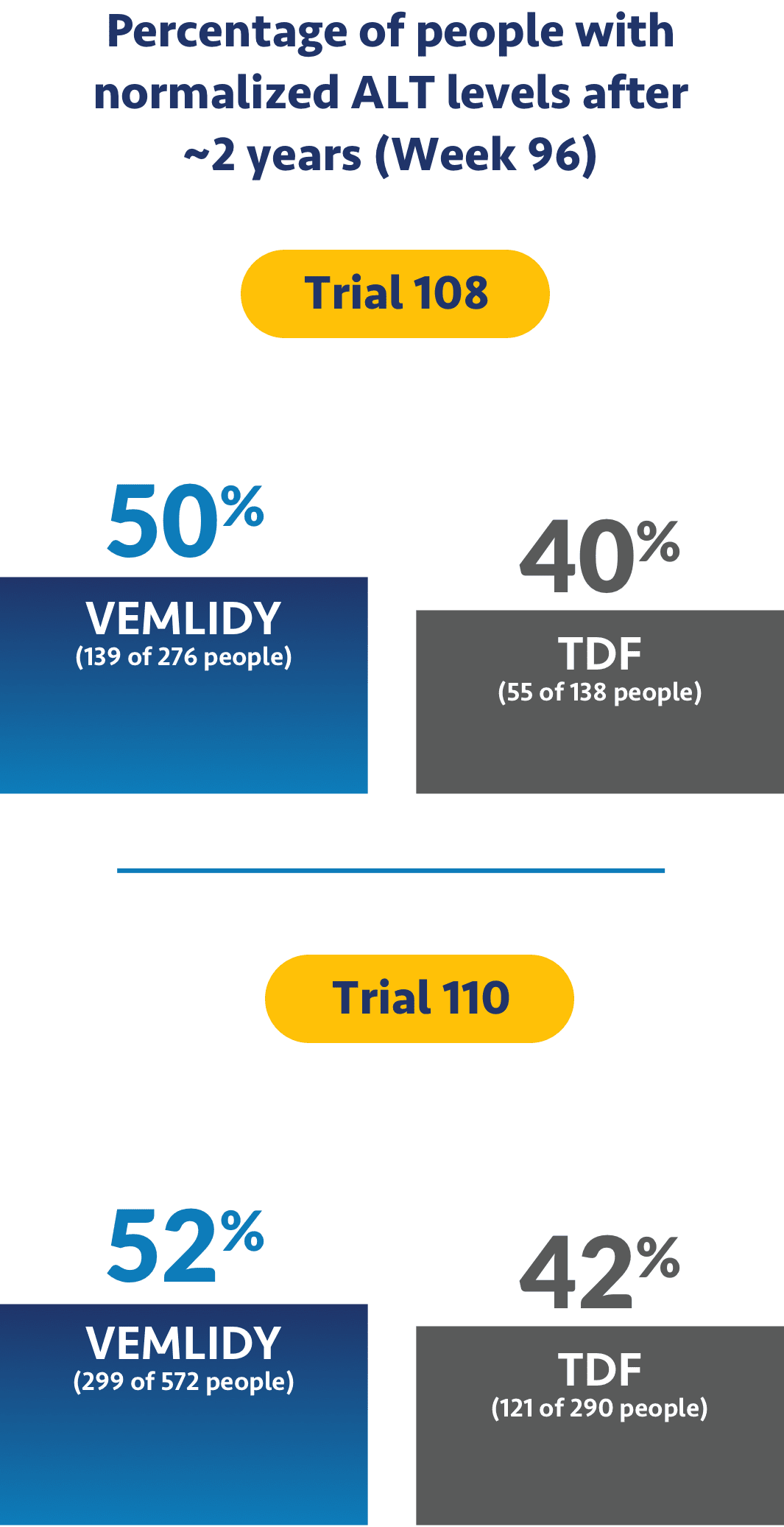
Ask your doctor about additional data points up to 8 years (Week 384).
VEMLIDY may cause severe liver problems, which in rare cases can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turn yellow, dark "tea-colored" urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.


